Stat News reports that the large, Phase 3 study testing a Covid-19 vaccine being developed by AstraZeneca and the University of Oxford at dozens of sites across the U.S. has been put on hold due to a suspected serious adverse reaction in a participant in the United Kingdom.
An individual familiar with the development said researchers had been told the hold was placed on the trial out of “an abundance of caution.”
Full statement from AstraZeneca:
“As part of the ongoing randomized, controlled global trials of the Oxford coronavirus vaccine, our standard review process triggered a pause to vaccination to allow review of safety data.
This is a routine action which has to happen whenever there is a potentially unexplained illness in one of the trials, while it is investigated, ensuring we maintain the integrity of the trials.
In large trials, illnesses will happen by chance but must be independently reviewed to check this carefully.
We are working to expedite the review of the single event to minimize any potential impact on the trial timeline. We are committed to the safety of our participants and the highest standards of conduct in our trials.“
AstraZeneca ADRs are down over 8% after hours…
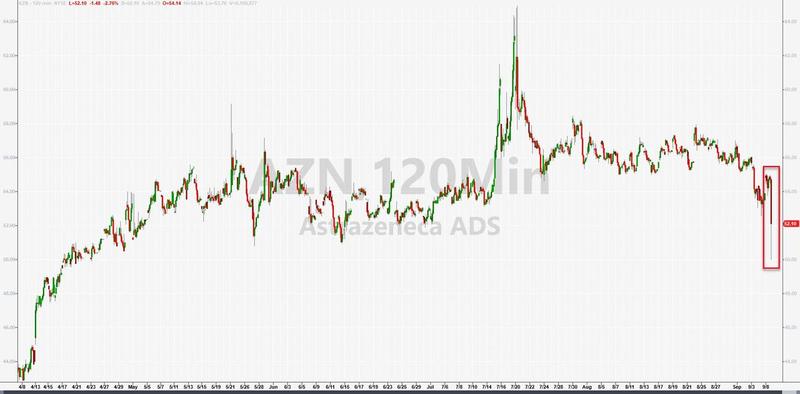
Perhaps of even greater import, Stat News reports that a second individual familiar with the matter, who also spoke on condition of anonymity, said the finding is having an impact on other AstraZeneca vaccine trials underway – as well as on the clinical trials being conducted by other vaccine manufacturers.
There are currently nine vaccine candidates in Phase 3 trials. AstraZeneca’s is the first Phase 3 Covid-19 vaccine trial known to have been put on hold.
Republished from ZeroHedge.com with permission









Sign up on lukeunfiltered.com or to check out our store on thebestpoliticalshirts.com.